Annex 1 : Are You Compliant?
The origin of Annex 1
Good Manufacturing Practices (GMP) are the practices required to comply with guidelines recommended by the agencies that control the authorisation and licensing of the manufacture and sale of food and beverages, cosmetics, pharmaceuticals, dietary supplements and medical devices.
These guidelines provide the minimum requirements that a manufacturer must meet to ensure that its products are of consistently high quality from batch to batch for their intended use. GMP is typically ensured through the effective use of a quality management system (QMS). The GMP contains 20 specific annexes.
The sterilisation and aseptic processing of sterile APIs are not covered, but should be performed in accordance with the principles and guidelines of GMP as laid down in national legislations and interpreted in the GMP Guide including its Annex 1.
The original version of Annex 1 was partially revised in 1993, 2003 and 2008. However there was never a complete review, although there were, since that time, significant changes in technologies, regulations and in the GMP by the adoption of the ICH* Q9 (Quality Risk Management) and Q10 (Pharmaceutical Quality System) guidelines.
Annex 1, which deals with the Manufacturing of Sterile Products, is one of the twenty annexes that make up the Good Manufacturing Practices (GMP) guide. Intended for companies in the pharmaceutical industry, Annex 1 shows the principles and guidelines for the aseptic/sterile manufacture of medicines for human and veterinary use.
The impact
Cleanroom clothing and its quality must be appropriate to the process and grade of the work area. According to Chapter 7, Paragraph 13 and 14 of the Annex 1, this now means:
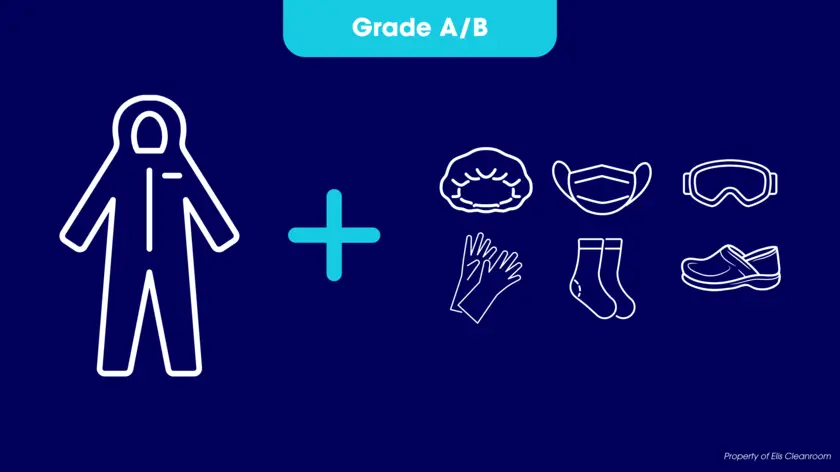
- Grade A / B: It becomes necessary to have a sterile cap, gloves, shoes and mask in combination with the sterile upper garments, as well as an undergarment which must be worn under a sterile suit.
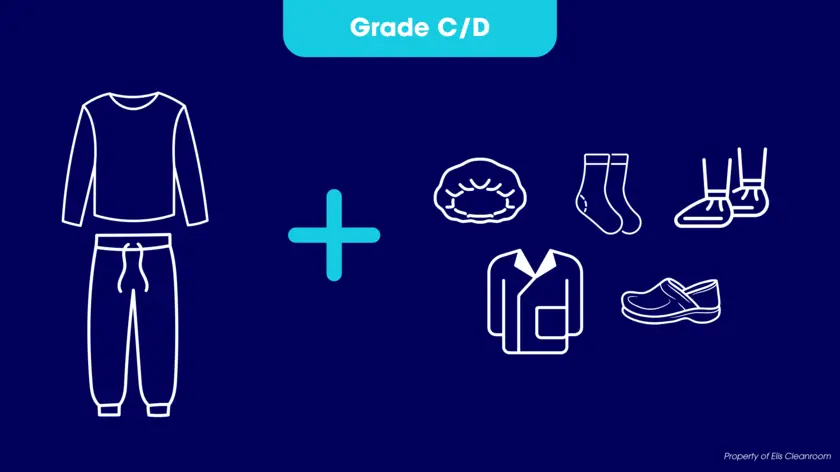
- Grade C: Operators must cover their hair, beard, and moustache. They should also wear disinfected trousers and shoes.
- Grade D: Hair, beard and moustache must be covered. A general protective suit and properly disinfected shoes (or overshoes) must be worn.
What other changes are expected?
It is detailed in Chapter 8, paragraph 46, that items that are not used immediately after sterilization must be stored in appropriately sealed packaging.
Clarification is also given regarding equipment that cannot be sterilized. Once disinfected, it must be protected to prevent recontamination (Chapter 8, paragraph 49)
Faced with these two major changes for your activity, Elis Cleanroom will support you with its autoclavable bags and covers, made to measure, thus adapting to your needs. Find them here:
Elis Cleanroom supports you in your compliance and offers you the right products for your needs.
Our products
Our expertise

At Elis Cleanroom, our cleanroom laundries are equipped with a Helmke drum for particle counting and we conduct thorough visual inspections to ensure Annex 1 compliance. Our validated processes include a set number of washes per garment and in-house sterilisation with our own autoclaves. All operations are handled by trained and validated operators. For any support with Annex 1 compliance, feel free to contact our team.
Subscribe to our newsletter

Want to know more? Contact us.
*Required fields
Contact details
Your legal name is the name that appears on your official documents.